A beginner’s guide to cell balancing in a home-made battery
Let’s start with some basics.
A cell is a container of chemicals that produces an electrical voltage. The voltage produced is a characteristic of the particular chemistry in use, for instance a lead-acid cell is nominally 2 V and a LFP cell is nominally 3.2 V. The bigger the container, the more chemicals it contains, and the larger the capacity, but the single cell voltage stays the same. You can get higher voltage by adding two cells together, but you can’t get a lower voltage by cutting a cell in half. Half a cell would be the same voltage but with half the capacity.
A battery is a bunch of cells connected together, either in series, parallel or both. The word ‘battery’ is used to refer to lots of similar things used together, such as a battery of guns, or a battery of cages for hens.
If you connect cells together in parallel, you are effectively creating a bigger cell. It will have the same voltage as a single cell but a higher capacity. Two cells in parallel will store twice as much energy as a single cell.
Connecting cells in series gives you a higher voltage. For instance the Brumby runs at 150 V, which means a lot of cells! The charger I use charges the entire battery at once, at 150 V, rather than each cell individually. The advantage of this is that I only need to connect to two points on the battery (the negative and positive terminals). The disadvantage is that the cell voltages can become unbalanced.
‘Balance’ in a battery is tricky to explain
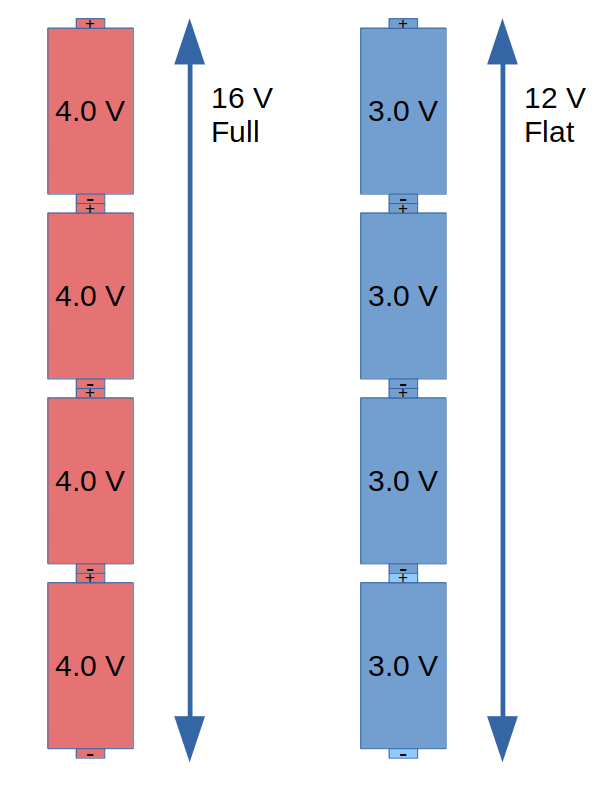
Let’s use the example of an NMC lithium cell. This cell is flat at 3.0 V and full at 4.0 V. The voltage on this type of cell varies fairly linearly with the state of charge, so that a half-full cell would be about 3.5 V. If we put four of these in series we get a battery that is 12 V when flat and 16 V when full. A suitable charger would charge this battery up and turn off at 16 V.
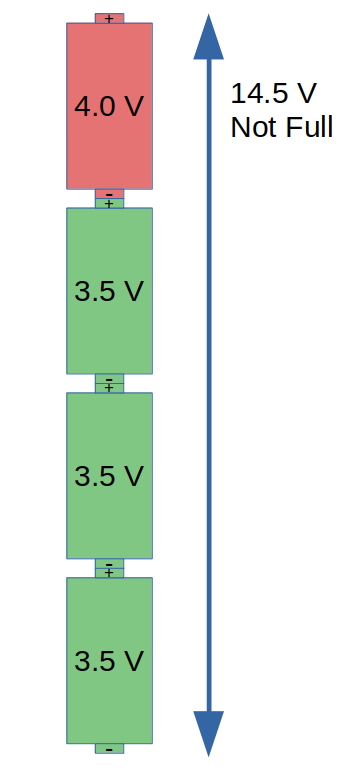
But what if one of the cells is not at the same voltage as the others? Let’s say that three of the cells were at 3.5 V, and the fourth was at 4.0 V. This adds up to 14.5 V total. A battery charger only ever sees the total voltage, so a 16 V charger would continue to charge this battery. As this battery charges past 14.5 V, the three cells are happy but the fourth cell goes above 4.0 V.
What happens when the cell is charged past its maximum voltage? It depends on the chemistry. Lead-acid cells aren’t too bad, they’ll gas out some hydrogen. But NMC cells will tend to get hot and might burn.

A similar problem happens when discharging. If we discharged our example battery down to 12 V then the lowest three cells would drop below 3.0 V.
Discharging a cell too low can cause permanent damage to the cell. For some chemistries the damage can be of the explosive kind!
So, you might be thinking that we should only build a battery with cells that are all the same charge. This is definitely a good start, and would probably do for a while. But as a battery is charged and discharged its component cells can become unbalanced over time. This is due to slight differences between cells, both in the way they are manufactured (perhaps one has slightly more chemical than another) and in their present conditions (perhaps one gets warmer than another).
Use a BMS to make sure you don’t damage imballanced cells
To know if cells are out of balance we need to monitor the voltage of each cell. At its simplest, a BMS (Battery Management System) will measure the voltage from each cell, and tell us if any get too high or too low. In our example, we’d set up the BMS to turn the charger off if any cell reached 4.0 V. We’d also set it up to stop discharge if any cell dropped to 3.0 V.
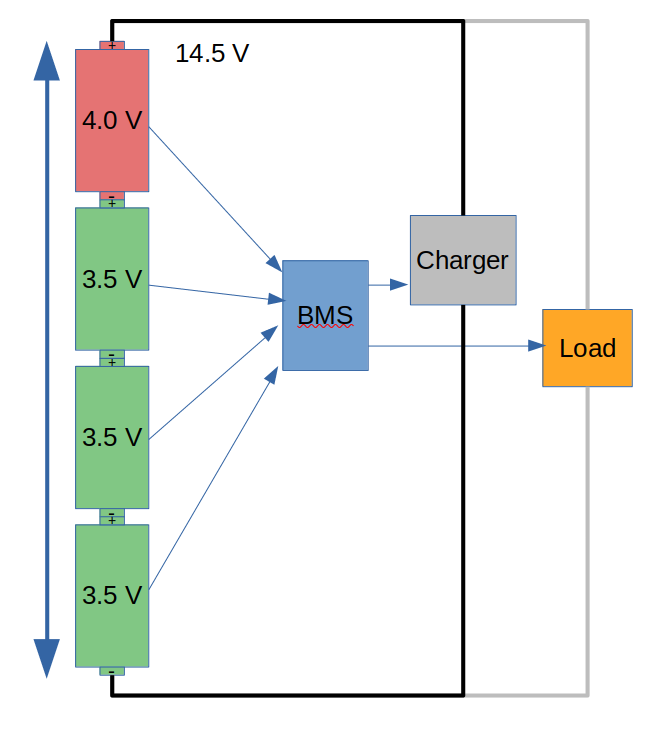
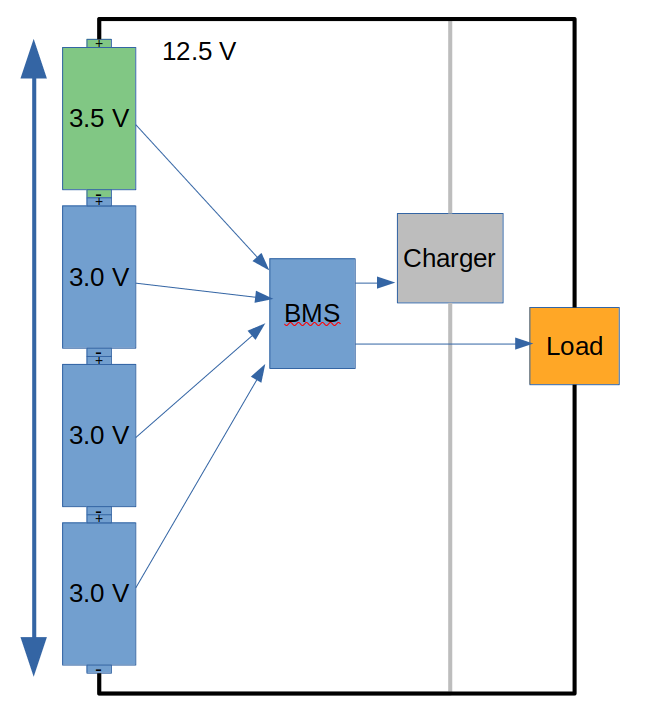
With our imbalanced example battery, the charger would be turned off by the BMS when the highest cell reached 4.0 V, in this case at a pack voltage of 14.5 V. Then during discharge, the BMS would stop things when the lowest reached 3.0 V, which is a pack voltage of 12.5 V. Our imbalanced battery is 12.5-14.5 V but an ideal battery is 12-16 V, so we’ve missed out on some storage capacity. Running an imbalanced battery like this is not going to damage anything, but there is potential storage in the battery that is not being used.
Use a BMS to balance your cells
To help regain capacity, a BMS will work towards balancing the cells within a battery. Different types of BMS will either take some charge from the higher cells, or add some charge to the lower cells, or do both. Arguably the simplest way is to take charge from the higher voltage cells, and that is the method described here.
The typical way to remove charge from a high voltage cell is to ‘burn it off’ by switching in a resistor across the positive and negative terminals of the cell. Such resistors are known as balancing, bleed, bypass or shunt resistors. The words ‘shunt’ and ‘bypass’ are a bit misleading, the extra capacity is not shunted anywhere, it is turned into heat and wasted.
The BMS knows the voltage of all cells. It finds the cell with the lowest voltage, and turns on the shunt for any cell higher than that voltage. Eventually, all the cells will come down to the same voltage as the lowest one, and the pack will be balanced. Next time we charge the battery it will have more capacity than it did when imbalanced.
Top balancing and bottom balancing
The example I gave is with NMC cells. These are simple to explain, since they have more-or-less linear discharge voltage between 3.0 and 4.0 V. But LFP cells (LiFePO4), and some other cell chemistries, have a more complicated voltage curve and need to be treated differently. The voltage of an LFP cell varies between about 2.7-3.6 V. But most of the capacity of a cell is between 3.1 and 3.3 V. A cell in this voltage range could be anything between 20-80% full, and using just voltage, there is no way of knowing exactly how full it is. This is annoying from our balancing point of view, since we can’t bleed off energy on the high cells if we don’t know which are the high cells!

There is a solution, and it uses the fact that the voltage of an LFP cell is useful when it is almost full or almost empty. When charging an LFP cell, the voltage slowly creeps up to 3.4 V, by which time it is about 95% full. It then rapidly increases up to 3.6 V, at which point it is full. This means that we can perform our balancing act when cells are above 3.4 V. This is called ‘top balancing’. The same can be done at the low end of battery capacity, in which case the term is ‘bottom balancing’. Bottom balancing doesn’t seem to be used for LFP though, so top balancing is what you’ll see.
The practicalities of top balancing mean that you can only balance when the battery is full. Balancing should be performed reasonably often to preserve the capacity of your battery, so a top balanced pack should be charged to 100% periodically. How often? It depends on your cells and your application. If all cells were made at the same time in the same factory, and are stored in the same place and treated the same, then a balance once a month will probably be fine. But if you have a battery in a car with some cells nicely insulated but some others out in the cold, then perhaps you need to balance more often. Discharging the battery to low levels more often will also increase the rate of imbalance.
People in this field often want a BMS that will balance a high current. I don’t see the point in this, since a low current over a long time will achieve the same thing, but with lower temperatures and cheaper components. Cells become imbalanced only slowly over time, so is not important to balance them quickly, you just need to be quicker than the forces of imbalance. The one exception to this rule is when you first put a battery together with very imbalanced cells. In that case a quick balance is nice, but a slow balance will still get the job done eventually.
But doesn’t balancing waste energy?
Another common concern is that energy is wasted with this type of balancing. This is correct, however we are talking about very small numbers. An example to illustrate this:
My Home Storage Battery is 440 AH, 48 V, with 16 LFP cells (3.2 V) in series. I get about 20 kWh from this battery, and most days it cycles at almost the full capacity. I find that if I balance the battery once a week, the balancing takes about 1 hour to complete.
My BMS can bypass up to 500 mA, and bypasses at 3.4 V, which equates to about 1.7 W per cell. Following the balancing logic above, the worst case scenario is that 15 of the 16 cells are balancing simultaneously, which adds up to 26 W. If this happens for 1 hour, this is 26 Wh wasted per week.
In perspective, this is 26 Wh out of 140,000 Wh (for the week), or 0.02% of total capacity. To put it another way, at 14c per kWh the wasted electricity would cost $0.005 per week, out of $19.60 stored.
In conclusion
Cell balancing is a topic that took me a long time to understand properly. In fact I don’t think I really understood the practice until after I’d built my own BMS and run a series of experiments with it. Hopefully my attempt to explain it here helps!
Watch this space for an article on how to make the DIY Low Cost BMS which can be used to balance your cells in a variety of situations.